We've all heard the saying, "It's not the heat, it's the humidity."
That statement couldn't be more true in Florida, especially during the summer months. So often the thermometer will read one thing and then when you walk outside, it feels so much hotter.
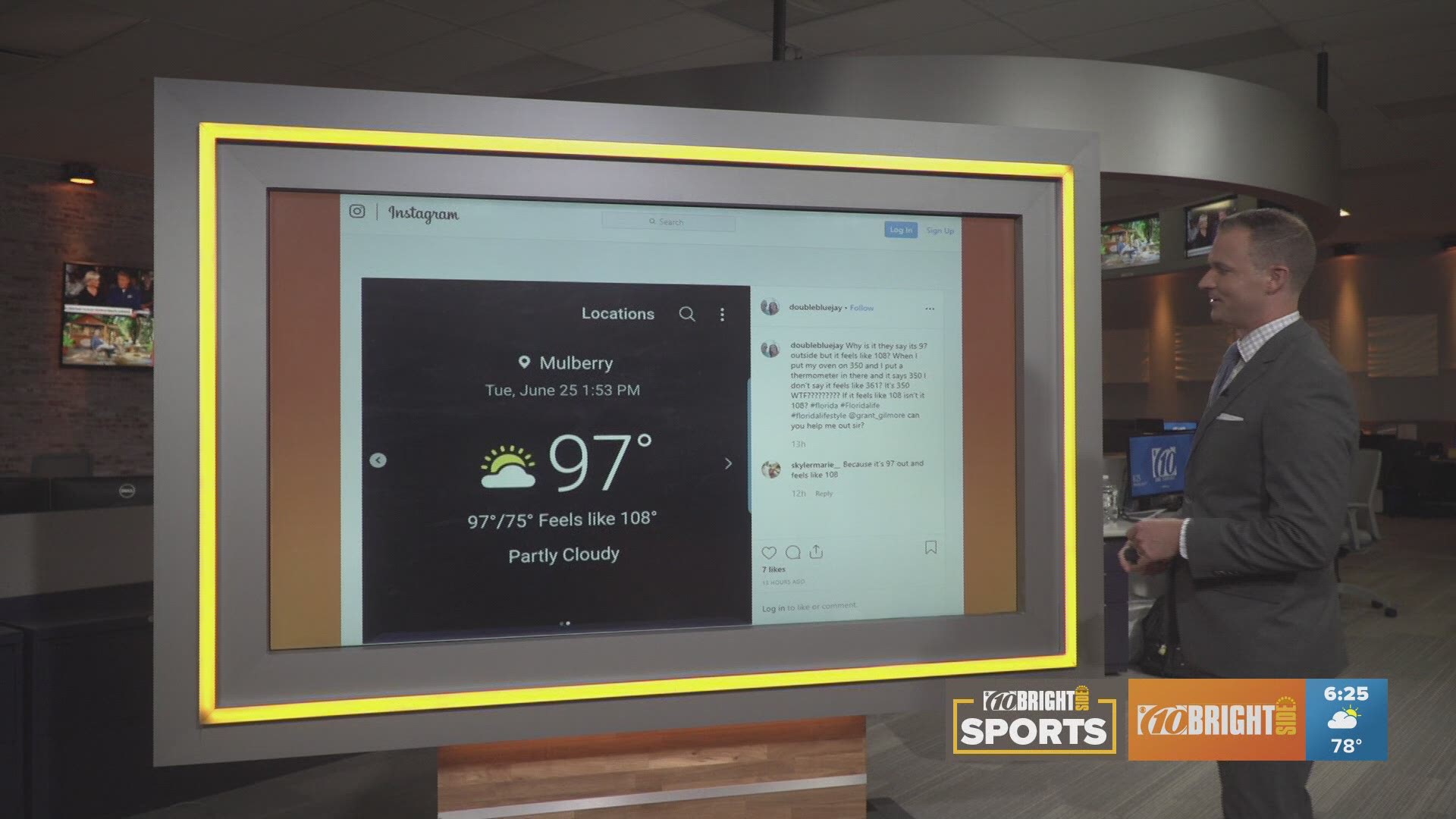
In the middle of a recent heat wave, a 10News viewer reached a boiling point and posted this on Instagram:
"Why is it they say its 97 outside but it feels like 108? When I put my oven on 350 and I put a thermometer in there and it says 350 I don't say it feels like 361? It's 350 WTF????????? If it feels like 108 isn't it 108? #florida #Floridalife #floridalifestyle @grant_gilmore can you help me out sir?"
The simple answer is the "feel-like temperature," the "Heat Index" or "Wind Chill" are apparent temperatures. In other words, it's what the temperature appears to be for a human due to the influence of other weather conditions such as wind speed, dew point, relatively humidity and amount of sunlight.
To explain this further, we have to define what temperature is in the first place. Temperature is the measure of the internal energy that a substance contains. The greater that internal energy, the faster atoms and molecules within that substance travel resulting in a higher temperature. A thermometer is able to measure the physical amount of heat within a substance.
The "feels-like temperature," specifically relating to when its values are greater than the actual temperature, is a measure of how hot it really feels for a human when the relative humidity is factored in. More specifically, when the atmosphere's level of saturation is considered.
People sweat in order to try to keep us from getting too hot. Sweating is an effective cooling bodily function because of the process of that sweat evaporating.
Let's back up, the process of a liquid transitioning to a gas is a phase change. All phase changes either release or absorb energy in order for that phase change to take place. In the case of evaporation, energy is absorbed, which results in a drop in temperature in the surrounding air.
Back to our sweat; it's not actually the sweat that cools us down, but rather the process of that sweat evaporating. The problem arises when your sweat cannot evaporate.
When there is a relatively large amount of moisture in the atmosphere, it becomes more difficult for liquid to evaporate. Basically, when the atmosphere gets closer to becoming fully saturated it cannot hold any more water.
This is where our comfort comes in. If the sweat on our skin is not able to evaporate, then it becomes more difficult for our body to cool itself. So, if your body can't cool like it normally would through sweating, a relatively cooler temperature will have the same effect on the body as if the temperature were actually hotter.
What other people are reading right now:
►Make it easy to keep up-to-date with more stories like this. Download the 10News app now.
Have a news tip? Email desk@wtsp.com, or visit our Facebook page or Twitter feed.